Many people are now discussing the potential of GLP-3 drugs as a promising avenue for weight loss.
You might be familiar with GLP-1 antagonists. These medications work by obstructing the action of Glucagon-Like Peptide-1, a hormone that typically encourages insulin release and curbs appetite, thus aiding in weight reduction. They are also advantageous for individuals with diabetes.
As interest in GLP-1 medications grows, another drug known as retatrutide has emerged, which could potentially be more effective.
In December, the pharmaceutical company Eli Lilly, known for producing Mounjaro and Zepbound, introduced a novel ‘triple antagonist’ called retatrutide, which has been informally dubbed ‘GLP-3.’
A study by Eli Lilly revealed that participants with obesity and knee osteoarthritis using retatrutide 12 mg experienced an average weight loss of 28.7 percent over 68 weeks.
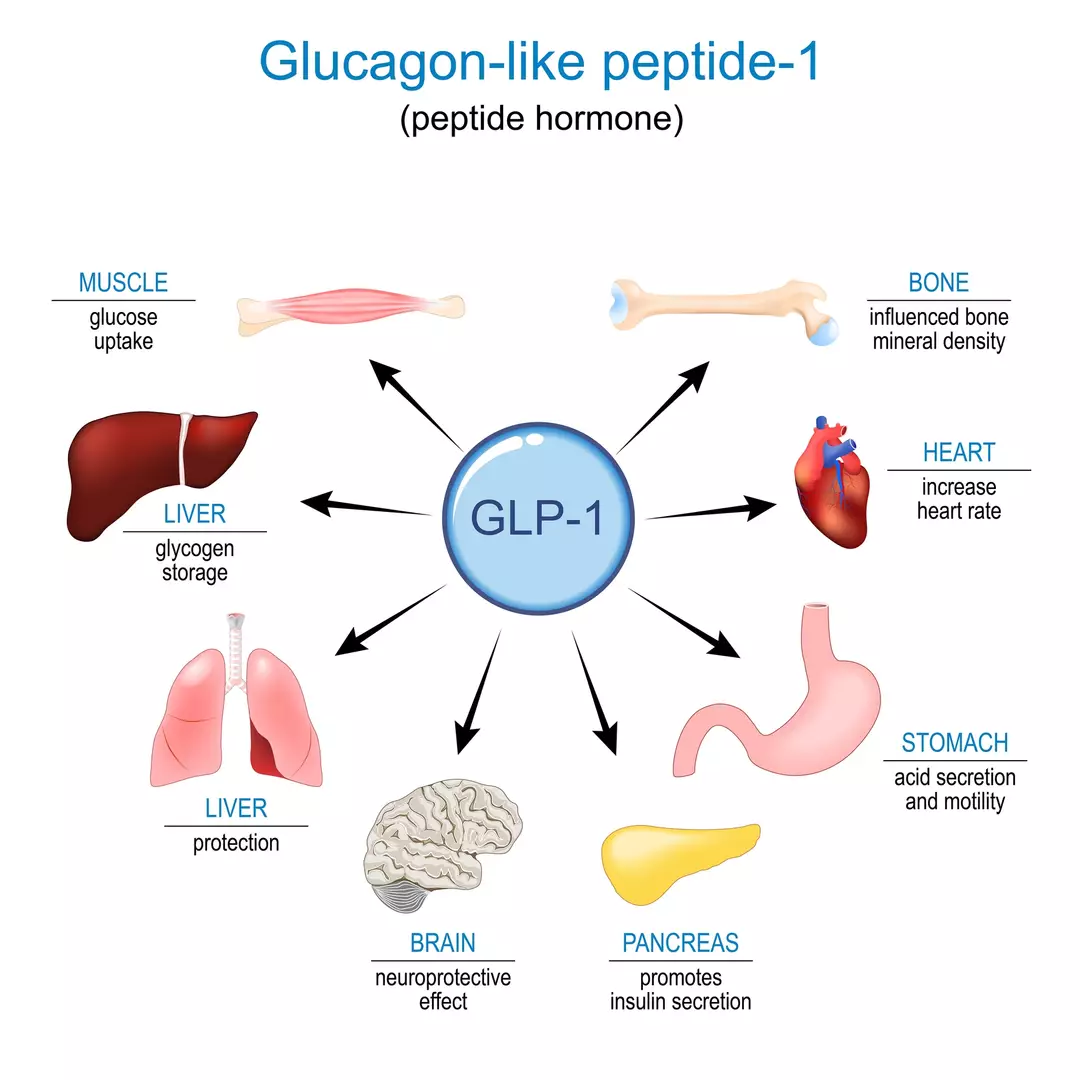
Eli Lilly describes this medication as an investigational once-weekly triple hormone receptor agonist.
“Retatrutide is a single molecule that activates the body’s receptors for glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), and glucagon,” according to the company.
While drugs like semaglutide only mimic GLP-1, tirzepatide mimics both GLP-1 and GIP.
The unique aspect of retatrutide is its ability to mimic glucagon as well, which aids in burning stored fat for energy and reduces body weight, as reported by The Independent.

In a specific trial known as the TRIUMPH-4 clinical trial, participants were either obese or overweight, did not have diabetes, and were suffering from osteoarthritis (OA) of the knee. The treatment was supplemented by a healthy diet and physical activity.
The study aimed to assess the ‘safety and efficacy of retatrutide once-weekly’ considering these conditions.
After 68 weeks, the study found that participants experienced ‘significant weight loss and improvements in pain and physical function at 68 weeks using both the efficacy and treatment-regimen estimands.’
Participants saw an average weight reduction of 28.7 percent (over 72lbs) and a decrease in pain by up to 4.5 points, measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain score.

Kenneth Custer, Ph.D., executive vice president and president of Lilly Cardiometabolic Health, commented: “We are encouraged by the results of TRIUMPH-4, which highlight the powerful effect of retatrutide, a first-in-class triple agonist, on body weight, pain and physical function.”
“With seven additional Phase 3 readouts expected in 2026, we believe retatrutide could become an important option for patients with significant weight loss needs and certain complications, including knee osteoarthritis.”
While Eli Lilly’s GLP-3 has not yet been licensed, its main competitor Novo Nordisk, the producer of Ozempic, announced in March 2025 that it had reached a licensing agreement with The United Laboratories, a Hong Kong-based pharmaceutical company.
Although retatrutide was not specifically named, the announcement referred to ‘a triple agonist of the receptors for GLP-1, GIP, and glucagon.’ The drug mentioned in the press release was UBT251.
The timeline for when the new GLP-3 medication will be available has not been disclosed.