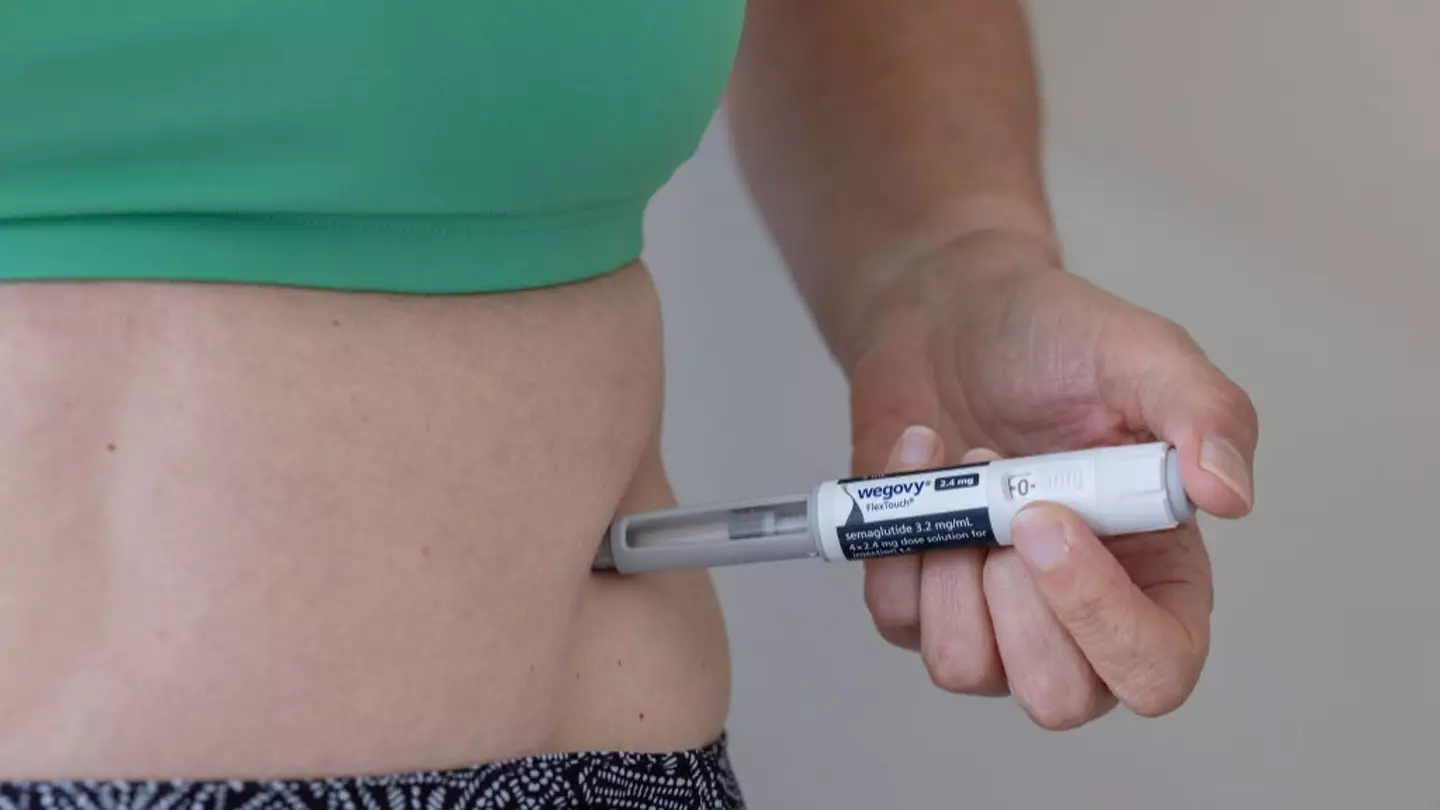
Health professionals have recently raised concerns about the effects on the body after ceasing the use of weight loss drugs.
Medications such as Wegovy and Mounjaro have gained significant popularity in the United States and globally, often used to manage type 2 diabetes or facilitate weight loss.
In the United States, approximately 15.5 million adults, which is around six percent of the adult population, are using these injectable drugs to help control obesity or diabetes, based on data from the Gallup National Health and Well-Being Index.
Despite the well-known side effects of GLP-1 drugs, which include impacts on sex drive, mental health, and skin sagging, health professionals are now cautioning about the consequences users might face after discontinuing the injections.
According to an independent committee from the National Institute for Health and Care Excellence (NICE), studies indicate that many individuals tend to regain the weight they lost after stopping the medication.
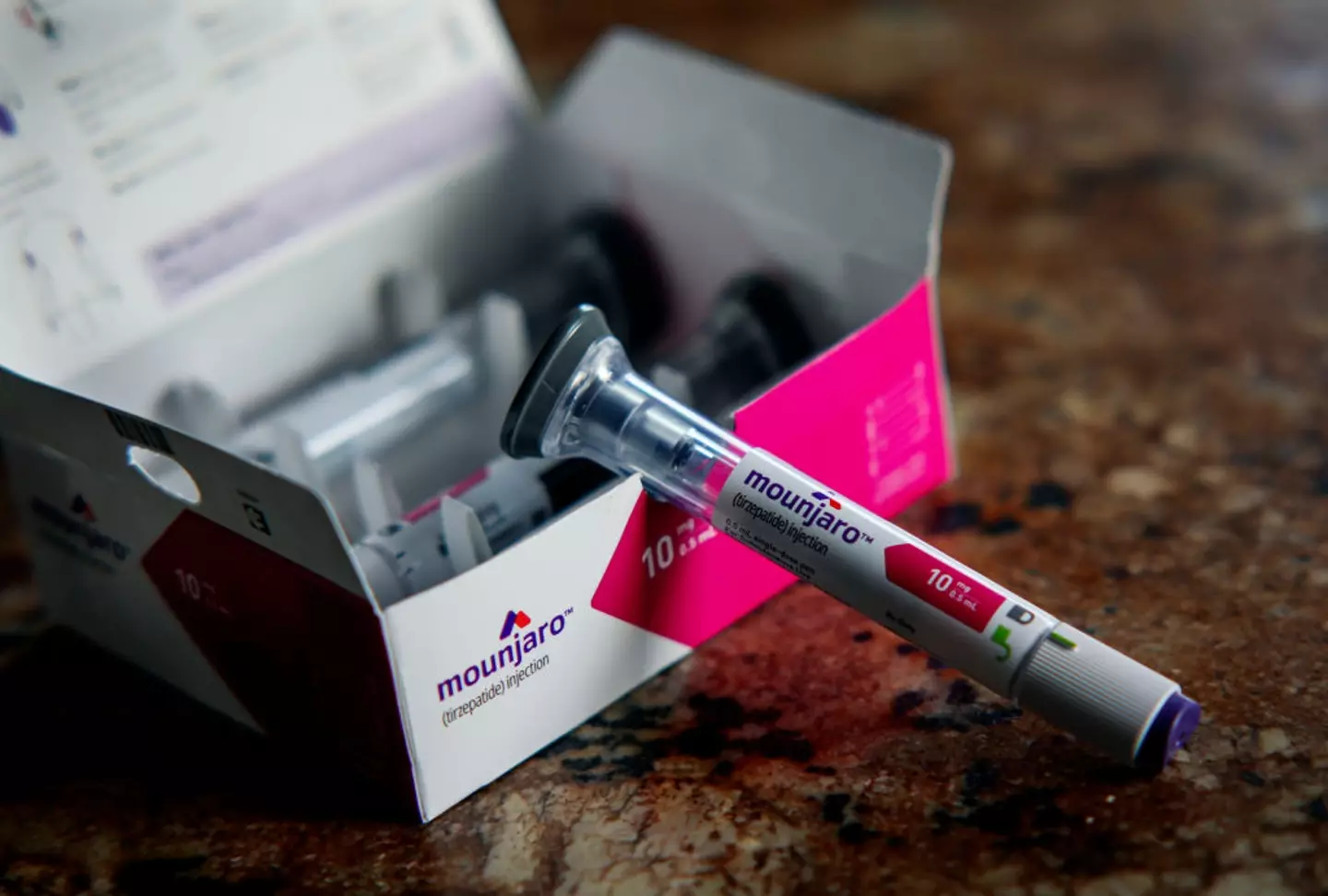
While these drugs are generally recommended alongside programs that emphasize healthy weight loss through dietary adjustments and increased physical activity, experts warn there is insufficient support to aid individuals in maintaining their weight once the treatment ends.
For instance, in a Wegovy (semaglutide) drug trial, participants regained approximately two-thirds of their original weight after ceasing the injections.
Similarly, a study on Mounjaro (tirzepatide) discovered that stopping the drug resulted in a significant regain of lost weight.
Another research conducted in China, which observed 2,466 patients following their cessation of GLP-1 medications, found ‘significant weight regain occurred eight weeks after discontinuation of AOMs [anti-obesity medications]’.
To bridge this gap and offer better support, the public health body is advocating for healthcare providers to deliver structured, personalized guidance and follow-up support to encourage lasting changes in daily habits.

This support may involve dieticians or nutritionists, peer support groups, and fitness activities. NICE also advises patients to learn how to modify their work or home environments, utilize self-monitoring tools, and seek wider support from online and local resources.
“Weight management is a long-term journey, not a short-term fix,” stated Dr. Rebecca Payne, Chair of NICE’s Quality Standards Advisory Committee.
“The evidence is clear that advice and support for maintaining weight after stopping medicines or completing behavioral interventions can help prevent weight regain and enable people to experience lasting benefits.”
Professor Jonathan Benger, deputy chief executive and chief medical officer at NICE, noted: “Successful weight management doesn’t end when medication stops or when someone completes a behavioral programme.”
“We know that the transition period after treatment is crucial, and people need structured support to maintain the positive changes they’ve made.”

He emphasized that the new standard should prompt service providers to develop a ‘continuity of care’ approach that transitions from a ‘sickness service’ to a ‘genuine health service focused on prevention’.
NICE has implemented this new standard across healthcare providers and commissioners in the UK ‘immediately’.
UNILAD has reached out to the manufacturers of Mounjaro, Eli Lilly and Company, and Wegovy, Novo Nordisk, for comments.