Discover the symptoms of scurvy, a condition once linked to pirates and sailors, which is seeing a resurgence due to weight loss medications.
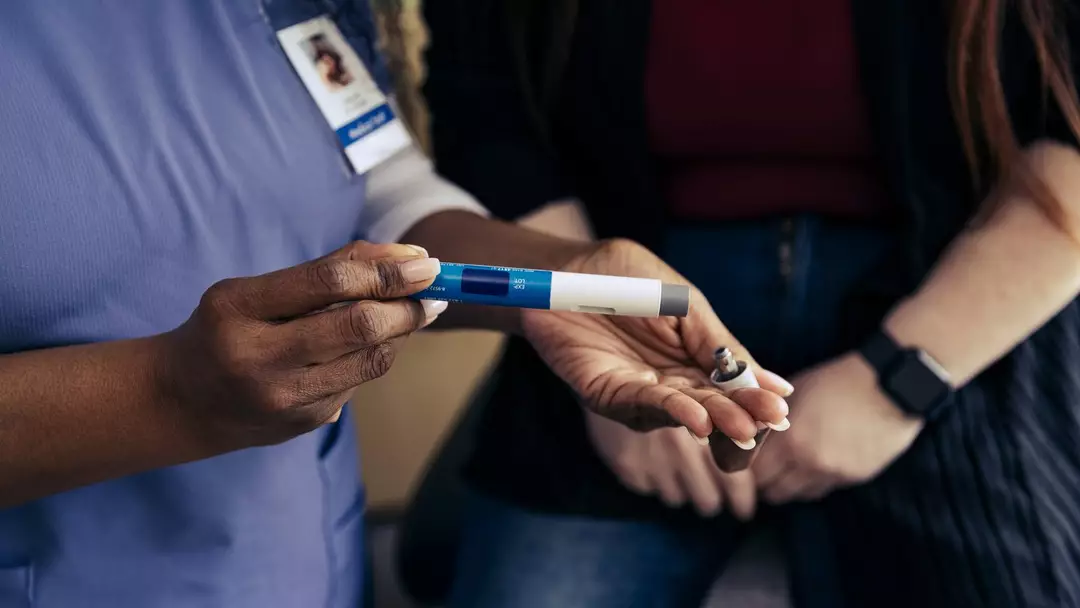
Weight loss drugs are currently in high demand, with a survey showing that one in eight adults have used GLP-1s for this purpose.
The side effects of these medications are frequently discussed, including a recent report about the slight risk of sudden blindness.
Recently, the potential side effect of scurvy has come under discussion, a disease more commonly associated with the pirate and sailor era hundreds of years ago.
Scurvy primarily results from a lack of sufficient vitamin C in the diet.
Mayo Clinic states: “Not eating enough fruits and vegetables is the main cause of the disease. Left untreated, scurvy can lead to bleeding gums, loosened teeth and bleeding under your skin.”

The NHS lists several symptoms of scurvy, including:
Experts are raising concerns about the nutritional deficiencies in individuals using GLP-1 weight loss drugs. Clare Collins, from the Newcastle School of Health Sciences, elaborates on her team’s findings.
Collins told The Australian Financial Review, “Only one [trial] had published what people ate. We wrote to all the authors, and got data from one more trial. This is being missed.
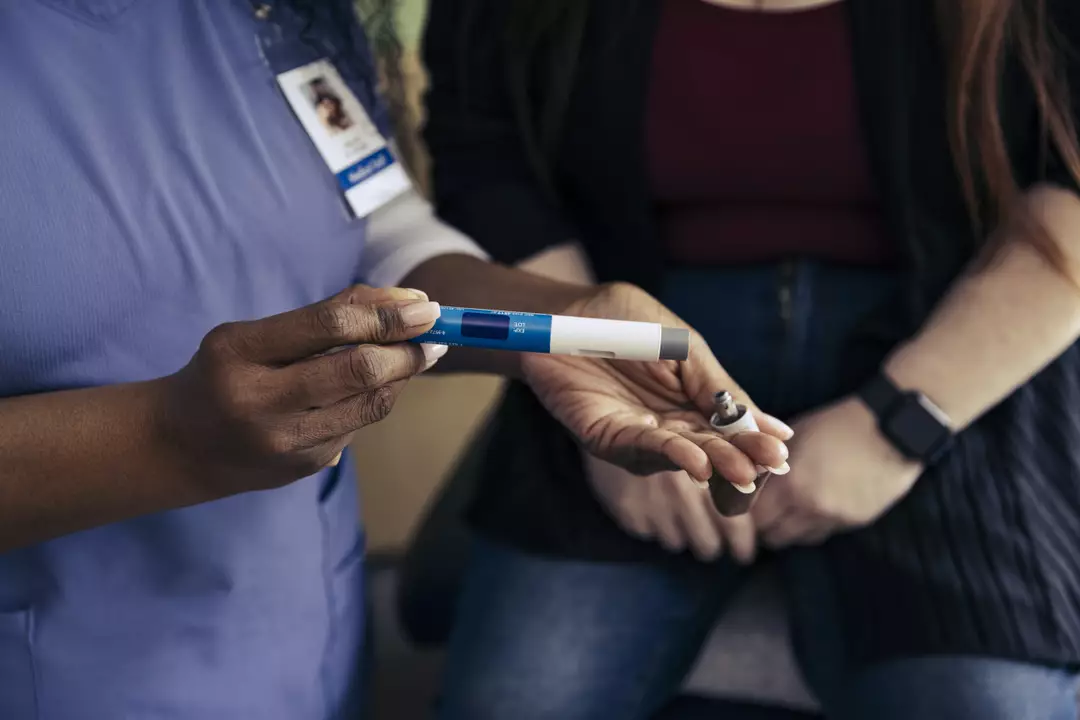
“It does not give you an opportunity on how it might impact their dietary intake. We know there are a lot of side effects. For such an expense it is a marked miss.”
“A reduction in body weight does not automatically mean the person is well, nourished or healthy. Nutrition plays a critical role in health and right now it’s largely missing from the evidence.”
Following these revelations, a representative for Novo Nordisk commented: “Given that the side effect to which you refer [scurvy] is not a recognised one that appears in the label, I don’t have anything specific I can share on this.
“I do have a more general statement though, and we recommend anyone experiencing any adverse events reports them to the MHRA to ensure relevant safety data is appropriately captured.”
For additional insights, Eli Lilly has been contacted for a statement.