Women who are using injections similar to Ozempic, Wegovy, or Mounjaro need to be extra cautious regarding their contraceptive methods.
GLP-1 medications, which include Wegovy and Mounjaro, have gained significant popularity due to their effectiveness in aiding weight loss. Similarly, Ozempic has also seen an increase in use, although it is primarily FDA-approved for treating Type-2 diabetes rather than for weight loss alone.
These medications function by imitating the body’s GLP-1 hormone, which plays a role in controlling blood sugar, appetite, and digestion speed, as explained by Harvard Health.
Tirzepatide, the active component in Mounjaro, operates similarly but also involves an additional hormone called glucose-dependent insulinotropic polypeptide (GIP).
The Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK has now issued a formal warning to individuals using these medications while also on birth control.
There has been an increase in reports of unexpected pregnancies among users on birth control, with the MHRA receiving over 40 reports of pregnancies among women taking these injections, resulting in what has been termed ‘Ozempic babies’ (source: ITV News).

The reported cases include 26 linked to Mounjaro, eight to semaglutide (the active ingredient in Ozempic and Wegovy), and nine to liraglutide (found in Saxenda and Victoza).
Specifically, it is believed that Mounjaro might reduce the effectiveness of the pill in individuals who are overweight.
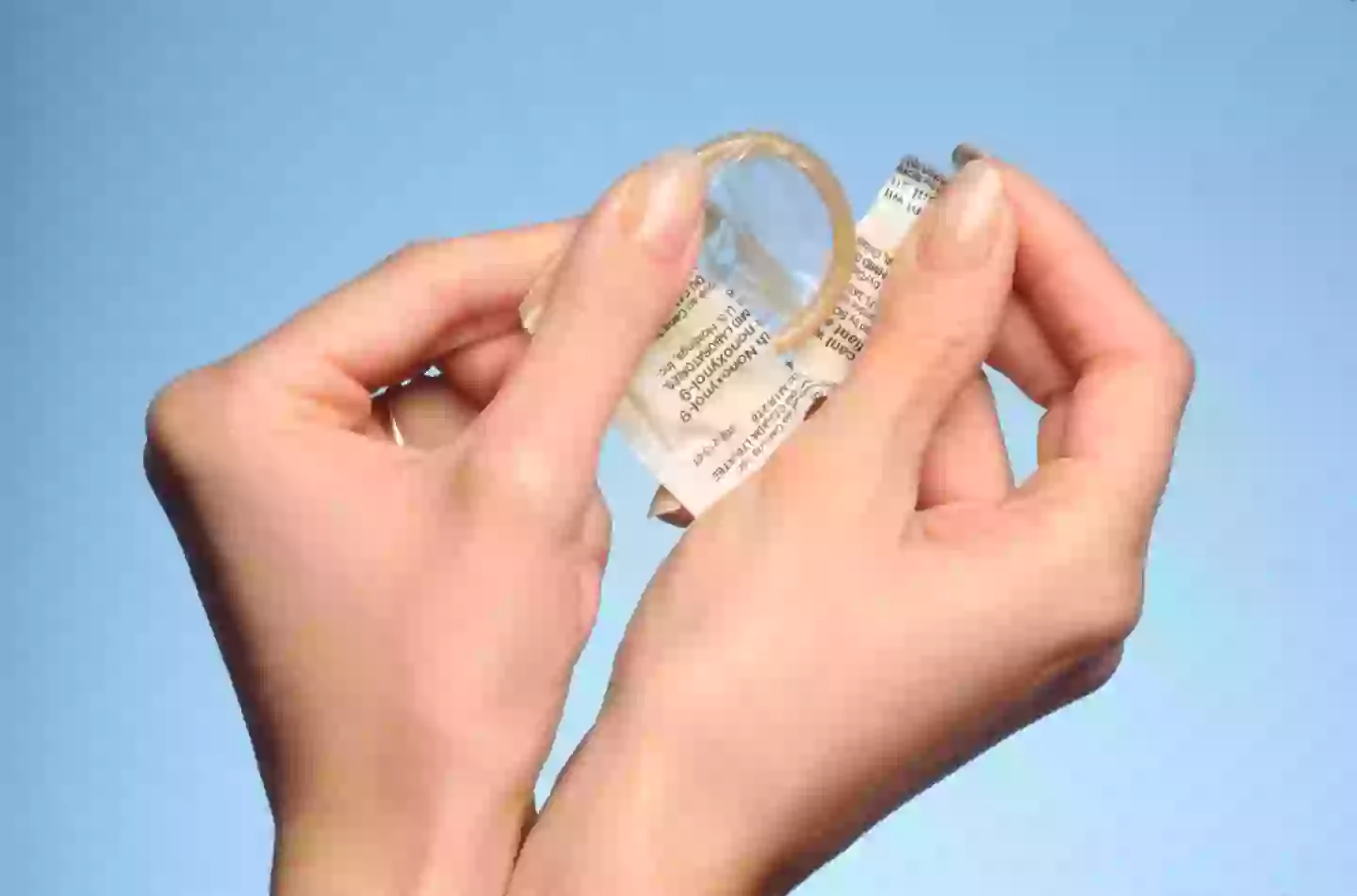
The MHRA in the UK recommends that women on the pill should also use condoms, particularly during the initial four weeks of treatment and following any increase in dosage.
They further caution that some women should wait up to two months after ceasing the injections before attempting to conceive, as the long-term effects on fertility and pregnancy remain uncertain.
The agency advises against using these drugs during pregnancy, while attempting to conceive, or during breastfeeding due to limited data on their safety for unborn children.
A Novo Nordisk representative informed UNILAD that there is limited data on the use of semaglutide in pregnant women to assess the risks of adverse outcomes for both mothers and fetuses.

The spokesperson stated, “Semaglutide injection (Ozempic®, Wegovy®) should be discontinued in women at least two months before a planned pregnancy due to the long washout period for semaglutide.”
They added that “Semaglutide should not be used during pregnancy. Women of childbearing potential are recommended to use contraception when treated with semaglutide.”
Moreover, in other pharmacology trials, semaglutide did not significantly affect the absorption of oral medications, including oral contraceptives like ethinylestradiol and levonorgestrel.
“Nonetheless, caution should be exercised when oral medications are concomitantly administered with semaglutide,” they advised.
Eli Lilly and Company, the manufacturer of Mounjaro, conveyed to UNILAD, “Patient safety is Lilly’s top priority, and we actively engage in monitoring, evaluating, and reporting safety information for all our medicines.”
The Summary of Product Characteristics for Mounjaro (tirzepatide) indicates that tirzepatide “has the potential to impact the rate of absorption of concomitantly administered oral medicinal products” and that this impact is most noticeable when initiating tirzepatide treatment.
The tirzepatide Patient Information Leaflet notes, “This medicine should not be used during pregnancy as the effects of this medicine on an unborn child are not known. If you are pregnant, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.”
The leaflet further recommends using contraception while on this medication. Women who are overweight or obese and using oral contraceptives should consider employing a barrier method (e.g., a condom) or transitioning to a non-oral contraceptive method for four weeks after starting Mounjaro and for four weeks after each dose increase.