A New York woman has taken legal action against Pfizer, claiming their birth control product led to her developing a sizable brain tumor.
Elizabeth Fleurisma, representing around 150 women, has filed a lawsuit against the pharmaceutical company after using their birth control for eight years.
At the age of 30, Fleurisma, a mother from Long Island, discovered a lime-sized brain tumor.
Fortunately, after enduring a 16-hour surgery followed by weeks of radiation treatment, most of the tumor was removed. However, a portion still remains in her skull.
In an interview with the New York Post, Fleurisma expressed that the tumor continues to affect her. “When I came out of surgery, when I came home, it’s almost like I didn’t even know my environment,” she said.
“Sometimes when I’m trying to speak, I’ll forget a word.”
Now nearing 33, Fleurisma noted, “It’s not easy healing from these things or getting back to where you left off.”
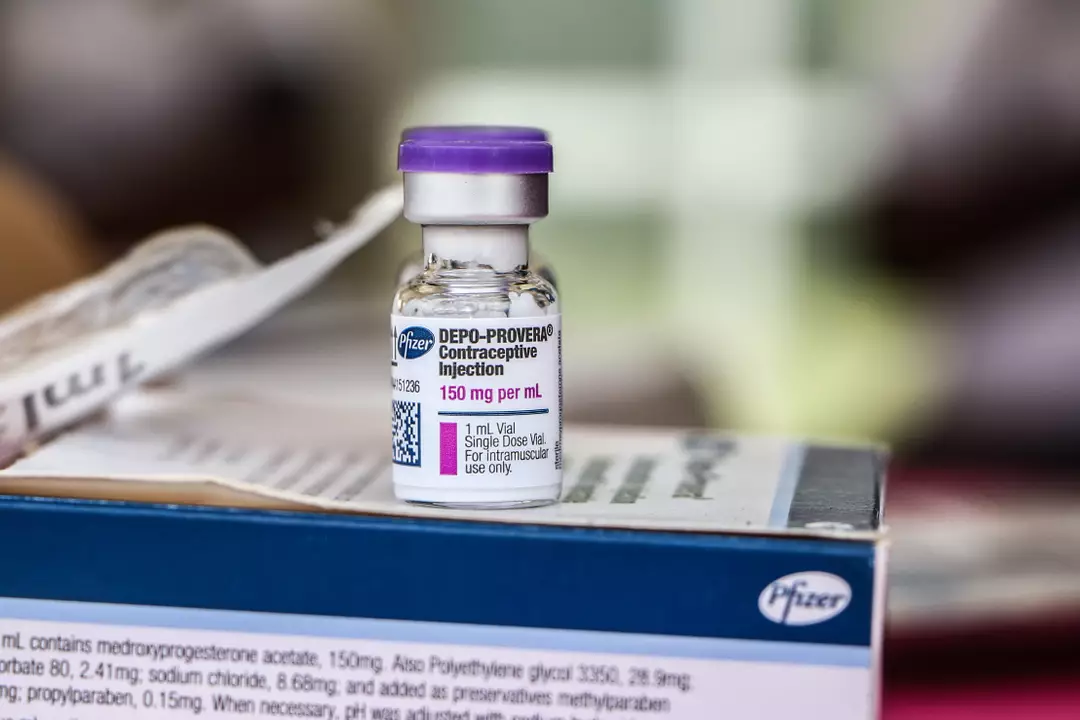
Her lawsuit against Pfizer, the makers of Depo-Provera, claims the company did not adequately inform users about the potential increased risk of brain tumors.
After giving birth at 18, Fleurisma opted for birth control but found it difficult to consistently take pills. She chose Depo-Provera, which requires an injection in the upper arm or buttock every three months, and used it for eight years.
This contraceptive method contains progestin, a synthetic hormone that prevents pregnancy by stopping ovulation and blocking sperm from reaching eggs.
The lawsuit contends that progestins significantly increase the likelihood of developing intracranial meningiomas, tumors originating from the meninges that shield the brain and spinal cord.
Nearly 1,500 separate lawsuits have been filed in federal court, with plaintiffs alleging that Pfizer was aware, or should have been aware, of the heightened risk of meningiomas but chose not to disclose it.
In response to Fleurisma’s lawsuit, Pfizer has stated it believes the claims are unfounded and intends to vigorously contest them.
“The Company stands behind the safety and efficacy of Depo-Provera, which has been used by millions of women worldwide and remains an important treatment option for women seeking to manage their reproductive health,” Pfizer asserted.
According to a Reuters report, Pfizer indicated that after evaluating research in 2023, which suggested a correlation between Depo-Provera and meningiomas, it requested the FDA to add a warning.
For further comment, UNILAD has reached out to Pfizer.

