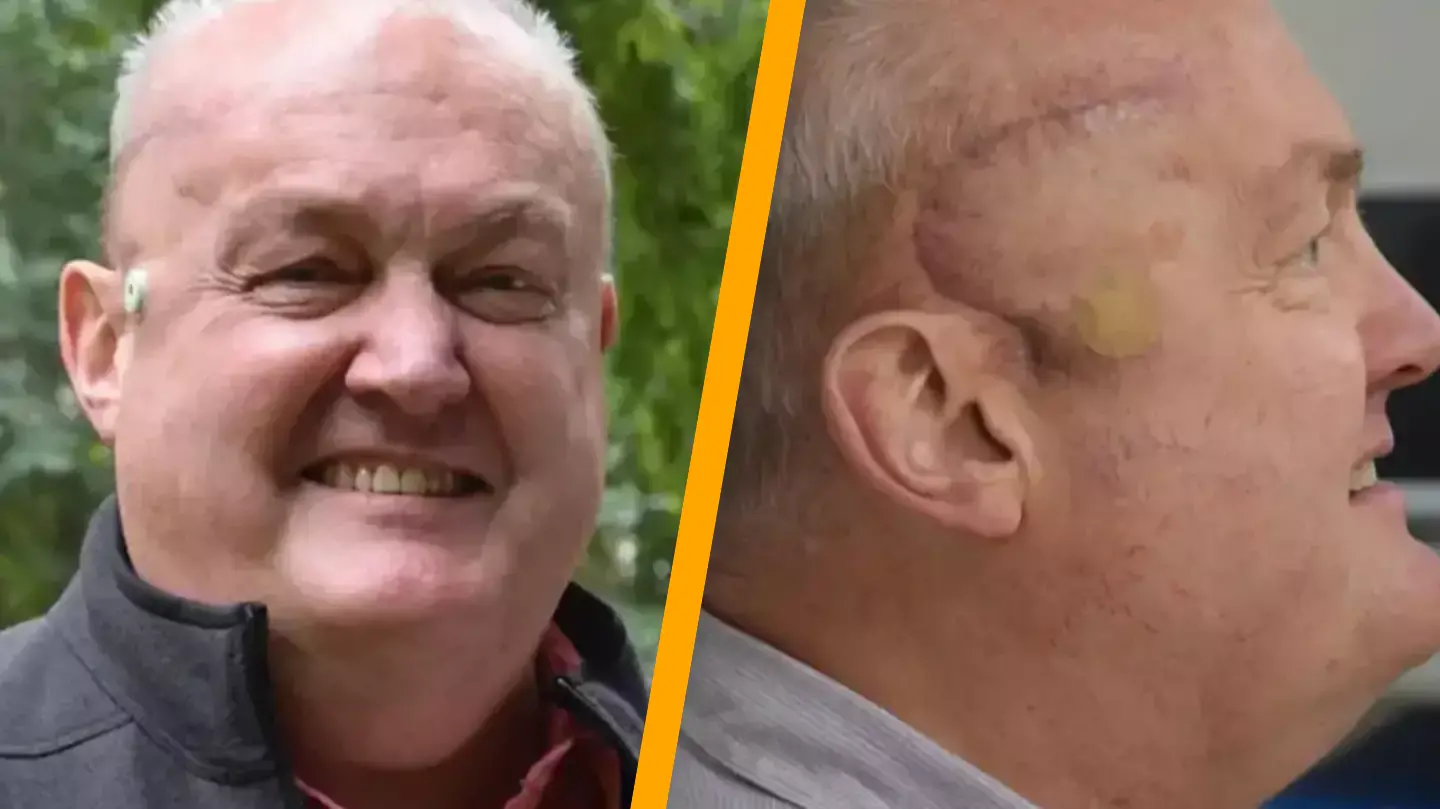
In the UK, a man who was given a prognosis of just one year to live has experienced a remarkable reduction in his brain tumor, which has shrunk by half after undergoing a pioneering cancer treatment.
Paul Read, aged 62, was diagnosed in December 2023 with glioblastoma, a form of cancer that usually comes with an 18-month survival expectation.
An innovative treatment was administered to Paul, leading to a ’50 percent’ decrease in his tumor as part of a groundbreaking trial.
This treatment was conducted at the University College London Hospitals NHS Foundation Trust (UCLH). Surgeons initially removed as much of the tumor as possible before placing an Ommaya reservoir device beneath Paul’s scalp.

Once the device was in place, doctors proceeded to inject low levels of radioactivity into the tumor once a week over a period of six weeks.
The injections were pivotal, aiming to destroy the cancer cells while sparing the surrounding healthy tissue.
At the conclusion of the treatment plan, brain scans astoundingly revealed that Paul’s tumor had ‘reduced in size by 50 percent’.
Dr. Paul Mulholland, a consultant medical oncologist and chief investigator leading the Glioblastoma Research Group at the UCL Cancer Institute, commented: “We’ve just gone through [Paul’s] scan results with him and his end of treatment scan shows a reduction in the tumor, which is really quite remarkable for somebody whose tumor is so aggressive.”
In light of the results, Paul expressed: “This trial was a lifeline, as the likelihood of survival, according to the data, was a year or less for me.”
Dr. Mulholland also mentioned in a hospital-issued statement: “We have been working with Ariceum Therapeutics for some years to develop this study. It will allow us to deliver low levels of radioactivity directly into the tumour of patients with recurrent glioblastoma.
“I’m very pleased that this clinical trial is now open. Potentially this is a very powerful approach and I’m already extremely happy with the results from the first patient.
“I’m also very proud at how my colleagues in neurosurgery and nuclear medicine have come together as a team to deliver a really novel trial.”
Fortunately, Paul reported only experiencing increased fatigue during his treatment, with no significant side effects.
The CITADEL-123 trial at UCLH was presented to Paul after his diagnosis, and he was ‘happy to explore anything’.
Reflecting on the trial, Paul remarked: “We are all dealt a hand of cards and you don’t know which ones you are going to get.”
Prior to starting the treatment, he stated: “It will be wonderful if this treatment helps me and if it doesn’t, it doesn’t… it may benefit someone else down the line.”
Currently, the same team that treated Paul is preparing to treat a second patient using the same method, with plans to expand the trial to 40 patients in its first phase.